Abstract
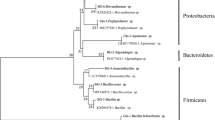
A Chlorella vulgaris ATCC 13482 culture was semi-continuously cultivated for 18 months in a 4-L photobioreactor and formed associated consortia with other symbionts. Three symbiotic bacterial strains were isolated on heterotrophic medium agar plates. Based on 16S rDNA analysis, they were found to show closest similarity to Pseudomonas alcaligenes, Elizabethkingia miricola and Methylobacterium radiotolerans. C. vulgaris was co-cultured with each bacterial strain, and it was found that the symbiotic bacterium Pseudomonas sp. had a growth-promoting effect on C. vulgaris while the other two inhibited algal growth. The interactions between C. vulgaris and Pseudomonas sp. were further investigated under different cultivation conditions. The co-culture resulted in 1.4 times greater algal cell concentration than that of C. vulgaris alone under photoautotrophic condition. In contrast, the algal cell concentration was lower in the co-culture compared with single algal culture when glucose was supplied in the medium (photoheterotrophic). Under both cultivation conditions, the number of Pseudomonas sp. increased at the beginning of experiment, and then decreased. However, the bacterial number decreased to almost zero under photoheterotrophic conditions, while the growth of bacteria went into a stationary phase under photoautotrophic conditions. The chlorophyll content in C. vulgaris cell was higher in co-culture than in single algal culture. Algal cells in photoautotrophic condition showed higher photosynthetic efficiency compared to those in photoheterotrophic condition. Extracellular organic carbon dissolved in the medium continuously increased under photoautotrophic condition. The mutualistic and competing relationships between C. vulgaris and symbiotic bacteria observed in this study could aid our understanding of algae–bacteria interactions in nature as well as broadening its practical applications.






Similar content being viewed by others
References
Barranguet C, Veuger B, Van Beusekom SAM, Marvan P, Sinke JJ, Admiraal W (2005) Divergent composition of algal–bacterial biofilms developing under various external factors. Eur J Phycol 40:1–8
Borde X, Guieysse B, Delgado O, Munoz R, Hatti-Kaul R, Nugier-Chauvin C, Patin H, Mattiasson B (2003) Synergistic relationships in algal–bacterial microcosms for the treatment of aromatic pollutants. Bioresour Technol 86:293–300
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Choi O, Das A, Yu CP, Hu ZQ (2010a) Nitrifying bacterial growth inhibition in the presence of algae and cyanobacteria. Biotechnol Bioeng 107:1004–1011
Choi SP, Nguyen MT, Sim SJ (2010b) Enzymatic pretreatment of Chlamydomonas reinhardtii biomass for ethanol production. Bioresour Technol 101:5330–5336
Cole JJ (1982) Interactions between bacteria and algae in aquatic ecosystems. Annu Rev Ecol Syst 13:291–314
Cosgrove J, Borowitzka MA (2011) Chlorophyll fluorescence terminology: an introduction. In: Suggett DJ, Prášil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic sciences. Springer, Dordrecht, pp 1–17
Croft MT, Lawrence AD, Raux-Deery E, Warren MJ, Smith AG (2005) Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 438:90–93
Czerpak R, Krotke A, Mical A (1999) Comparison of stimulatory effect of auxins and cytokinins on protein, saccharides and chlorophylls content in Chlorella pyrenoidosa Chick. Pol Arch Hydrobiol 46:71–82
de-Bashan LE, Moreno M, Hernandez JP, Bashan Y (2002) Removal of ammonium and phosphorus ions from synthetic wastewater by the microalgae Chlorella vulgaris coimmobilized in alginate beads with the microalgae growth-promoting bacterium Azospirillum brasilense. Water Res 36:2941–2948
DellaGreca M, Zarrelli A, Fergola P, Cerasuolo M, Pollio A, Pinto G (2010) Fatty acids released by Chlorella vulgaris and their role in interference with Pseudokirchneriella subcapitata: experiments and modelling. J Chem Ecol 36:339–349
Fukami K, Nishijima T, Ishida Y (1997) Stimulative and inhibitory effects of bacteria on the growth of microalgae. Hydrobiologia 358:185–191
Gong YM, Hu HH, Gao Y, Xu XD, Gao H (2011) Microalgae as platforms for production of recombinant proteins and valuable compounds: progress and prospects. J Ind Microbiol Biotechnol 38:1879–1890
Gonzalez LE, Bashan Y (2000) Increased growth of the microalga Chlorella vulgaris when coimmobilized and cocultured in alginate beads with the plant-growth-promoting bacterium Azospirillum brasilense. Appl Environ Microbiol 66:1527–1531
Guillard RRL (2005) Purification methods for microalgae. In: Andersen RA (ed) Algal culturing techniques. Elsevier Academic Press, Burlington, pp 117–132
Gurung TB, Urabe J, Nakanishi M (1999) Regulation of the relationship between phytoplankton Scenedesmus acutus and heterotrophic bacteria by the balance of light and nutrients. Aquat Microb Ecol 17:27–35
Hernandez JP, De-Bashan LE, Rodriguez DJ, Rodriguez Y, Bashan Y (2009) Growth promotion of the freshwater microalga Chlorella vulgaris by the nitrogen-fixing, plant growth-promoting bacterium Bacillus pumilus from and zone soils. Eur J Soil Biol 45:88–93
Hodoki Y, Ohbayashi K, Kobayashi Y, Okuda N, Nakano S (2011) Temporal variation in cyanobacteria species composition and photosynthetic activity in experimentally induced blooms. J Plankton Res 33:1410–1416
Huo YX, Cho KM, Rivera JGL, Monte E, Shen CR, Yan Y, Liao JC (2011) Conversion of proteins into biofuels by engineering nitrogen flux. Nat Biotechnol 29:346–351
Hyenstrand P, Burkert U, Pettersson A, Blomqvist P (2000) Competition between the green alga Scenedesmus and the cyanobacterium Synechococcus under different modes of inorganic nitrogen supply. Hydrobiologia 435:91–98
Imai I, Ishida Y, Sakaguchi K, Hata Y (1995) Algicidal marine-bacteria isolated from northern Hiroshima bay, Japan. Fisheries Sci 61:628–636
Jung SW, Kim BH, Katano T, Kong DS, Han MS (2008) Pseudomonas fluorescens HYK0210-SK09 offers species-specific biological control of winter algal blooms caused by freshwater diatom Stephanodiscus hantzschii. J Appl Microbiol 105:186–195
Lee BK, Katano T, Kitamura SI, Oh MJ, Han MS (2008) Monitoring of algicidal bacterium, Alteromonas sp. strain A14 in its application to natural Cochlodinium polykrikoides blooming seawater using fluorescence in situ hybridization. J Microbiol 46:274–282
Lee YK, Shen H (2004) Basic culturing techniques. In: Richmond A (ed) Handbook of microalgal culture: biotechnology and applied phycology. Blackwell Publishing, Oxford, pp 40–56
Liu JQ, Lewitus AJ, Kempton JW, Wilde SB (2008) The association of algicidal bacteria and raphidophyte blooms in South Carolina brackish detention ponds. Harmful Algae 7:184–193
Mazur H, Konop A, Synak R (2001) Indole-3-acetic acid in the culture medium of two axenic green microalgae. J Appl Phycol 13:35–42
Mouget JL, Dakhama A, Lavoie MC, de la Noue J (1995) Algal growth enhancement by bacteria — is consumption of photosynthetic oxygen involved. Fems Microbiol Ecol 18:35–43
Munoz R, Köllner C, Guieysse B, Mattiasson B (2004) Photosynthetically oxygenated salicylate biodegradation in a continuous stirred tank photobioreactor. Biotechnol Bioeng 87:797–803
Munoz R, Guieysse B (2006) Algal-bacterial processes for the treatment of hazardous contaminants: a review. Water Res 40:2799–2815
Ortiz-Marquez JCF, Nascimento MD, Dublan MD, Curatti L (2012) Association with an ammonium-excreting bacterium allows diazotrophic culture of oil-rich eukaryotic microalgae. Appl Environ Microbiol 78:2345–2352
Paerl HW (1976) Specific associations of blue-green algae Anabaena and Aphanizomenon with bacteria in freshwater blooms. J Phycol 12:431–435
Parmar A, Singh NK, Pandey A, Gnansounou E, Madamwar D (2011) Cyanobacteria and microalgae: a positive prospect for biofuels. Bioresour Technol 102:10163–10172
Paul C, Pohnert G (2011) Interactions of the algicidal bacterium Kordia algicida with diatoms: regulated protease excretion for specific algal lysis. Plos One 6:e21032
Pratt R, Daniels TC, Eiler JJ, Gunnison JB, Kumler WD, Oneto JF, Strait LA, Spoehr HA, Hardin GJ, Milner HW, Smith JH, Strain HH (1944) Chlorellin, an antibacterial substance from Chlorella. Science 99:351–352
Rier ST, Stevenson RJ (2002) Effects of light, dissolved organic carbon, and inorganic nutrients on the relationship between algae and heterotrophic bacteria in stream periphyton. Hydrobiologia 489:179–184
Rivas MO, Vargas P, Riquelme CE (2010) Interactions of Botryococcus braunii cultures with bacterial biofilms. Microbial Ecol 60:628–635
Sapp M, Schwaderer AS, Wiltshire KH, Hoppe HG, Gerdts G, Wichels A (2007) Species-specific bacterial communities in the phycosphere of microalgae? Microbial Ecol 53:683–699
Sarmento H, Gasol JM (2012) Use of phytoplankton-derived dissolved organic carbon by different types of bacterioplankton. Environ Microbiol 14:2348–2360
Seyedsayamdost MR, Carr G, Kolter R, Clardy J (2011) Roseobacticides: small molecule modulators of an algal–bacterial symbiosis. J Am Chem Soc 133:18343–18349
Su YY, Mennerich A, Urban B (2012) Synergistic cooperation between wastewater-born algae and activated sludge for wastewater treatment: influence of algae and sludge inoculation ratios. Bioresour Technol 105:67–73
Suminto, Hirayama K (1997) Application of a growth-promoting bacteria for stable mass culture of three marine microalgae. Hydrobiologia 358:223–230
Tarakhovskaya ER, Maslov YI, Shishova MF (2007) Phytohormones in algae. Russ J Plant Physio 54:163–170
Terauchi AM, Peers G, Kobayashi MC, Niyogi KK, Merchant SS (2010) Trophic status of Chlamydomonas reinhardtii influences the impact of iron deficiency on photosynthesis. Photosynth Res 105:39–49
Tison DL, Lingg AJ (1979) Dissolved organic matter utilization and oxygen uptake in algal–bacterial microcosms. Can J Microbiol 25:1315–1320
Vance BD (1987) Phytohormone effects on cell-division in Chlorella-pyrenoidosa chick (TX-7-11-05) (Chlorellaceae). J Plant Growth Regul 5:169–173
Watanabe K, Takihana N, Aoyagi H et al (2005) Symbiotic association in Chlorella culture. Fems Microbiol Ecol 51:187–196
Zhong WH, Li YX, Sun KD, Jin J, Li X, Zhang F, Chen J (2011) Aerobic degradation of methyl tert-butyl ether in a closed symbiotic system containing a mixed culture of Chlorella ellipsoidea and Methylibium petroleiphilum PM1. J Hazard Mater 185:1249–1255
Acknowledgments
We acknowledge the funding support under the grant number R302000011112 and the research scholarship for Mr. Zhi Guo from the National University of Singapore. This research is also supported by the National Research Foundation Singapore under its CREATE Programme (Singapore–Peking University Research Centre for a Sustainable Low Carbon Future).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
SEM images of C. vulgaris and symbionts, pH and DO profiles under photoautotrophic and photoheterotrophic conditions are given in the Online Resource.
ESM 1
(DOC 679 kb)
Rights and permissions
About this article
Cite this article
Guo, Z., Tong, Y.W. The interactions between Chlorella vulgaris and algal symbiotic bacteria under photoautotrophic and photoheterotrophic conditions. J Appl Phycol 26, 1483–1492 (2014). https://doi.org/10.1007/s10811-013-0186-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-013-0186-1